CDMO Services
With a one-stop RNA drug discovery and manufacturing platform, Immorna is committed to providing convenient and reliable development and manufacturing services for RNA therapeutics and RNA vaccines. Immorna's products have received multiple IND approvals from NMPA and FDA, and are in clinical trials in China and the US. Immorna has a number of proprietary technology platforms, including RNA "dumbbell" structures that enable single-stranded multivalent design, clinically validated self-replicating vectors, unique patented multi-component targeted LNP design, stable lipid carrier process formulation, and domestic RTU vectors with long term thermal stability. Biopharmaceutical companies use Immorna as an important supplier and partner for RNA design and liposome encapsulation to complete the transition from early stage research to clinical trials.
Custom Services>Immorna process development and production services
01
Sequence Design
02
Plasmid Preparation
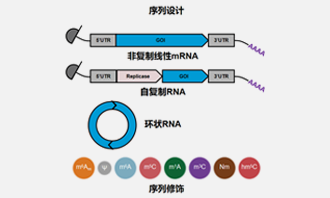
03
In vitro transcription of IVT

04
RNA purification
05
Vector Design

06
Encapsulation
07
Filling delivery
08
Analytical Methods
09
Stability Studies
10
IND Filings
Service Guarantee

Project Experience
> We have successfully completed Phase 1/2 clinical drug supply for 3 trials and obtained a total of 4 FDA IND approvals and 2 CDE IND approvals.
> We have accumulated experience in completing more than 40 Tox and GMP batches.
> Our clientele spans academic institutions, biotech companies, and renowned pharmaceutical enterprises.
Intellectual Property Protection
> We have clear confidentiality agreements in place to safeguard our clients' intellectual property.
> Our comprehensive project management mechanisms are designed to reduce project risks.
> A strict three-tiered account authorization system is enforced to effectively manage equipment operation permissions.
> We ensure stable electronic data storage media with robust permission management, ensuring the security and integrity of the data throughout its lifecycle.
> We employ standard IT techniques, including physical segregation of the office network from the production network, USB access control, employee internet usage management, and restrictions on user access to client information, preventing queries, copying, exporting, and similar actions.

Quality Management
> We have established a robust quality system in compliance with GMP requirements, following ICH guidelines, and meeting the expectations of regulatory bodies such as FDA, EMA, and NMPA. We maintain control over more than 350 existing SOPs and batch production record documents.
> We have implemented a comprehensive supplier management system.
> Our standard EMS monitoring system enables real-time tracking of the stability of key parameters like temperature, humidity, and pressure differentials within production areas, cleanrooms, or storage facilities. This ensures the stability and safety of our production environment.
> We adhere to a standardized computerized confirmation and validation system.
Original liquid quality control
Stability of 6 months at -60℃ storage condition
RNA Platform Examples
Traditional non-self-replicating RNA
> Stable cap rate over 98%;
> mRNA purity exceeds 90%;
> maintained good batch-to-batch consistency
Non-self-replicating mRNA stock mRNA purity, capping rate, Poly(A) tail length distribution
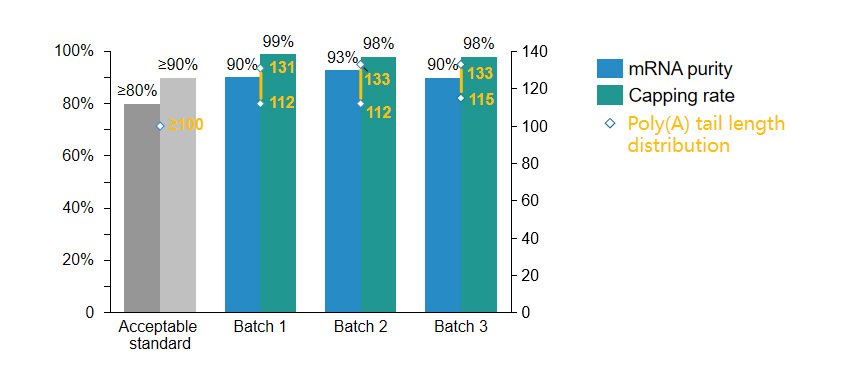
Non-self-replicating mRNA stock mRNA purity, capping rate, Poly(A) tail length distribution
Examples of RNA platforms
Self-replicating RNA synthesis platform
> Capping rate ≥90%
> mRNA purity ≥70%
Self-replicating RNA stock solution mRNA purity, capping rate, Poly(A) tail length distribution
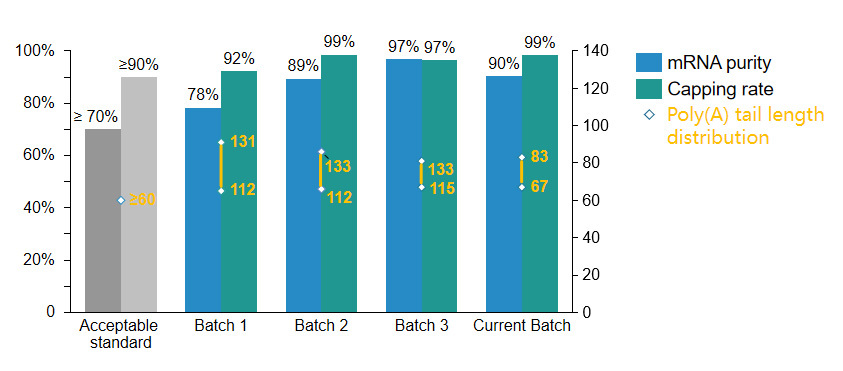
Self-replicating RNA stock mRNA purity, capping rate, Poly(A) tail length distribution
|
Test Items |
Test Methods |
|
Appearance |
Visual inspection |
|
pH |
Potentiometry |
|
mRNA concentration |
UV/Ribogreen |
|
5' capping efficiency |
LC-MS |
|
Poly A tail distribution |
LC-MS/CE |
|
mRNA integrity |
CE/HPLC/Agarose gel |
|
Sequencing |
Sanger |
|
dsRNA residues |
ELISA/Dot-blot |
|
IVTase residue (total protein) |
ELISA/NanoOrange |
|
DNA template residue detection |
ddPCR |
|
Sterility testing |
Pharmacopeial methods |
|
Endotoxin Testing |
Pharmacopeial methods |
Formulation Quality Control
Patented heat-stabilized delivery system for liquid dosage forms, RTU LNP technology, achieving stability verification of over 18 months of storage at 2-8°C.

Non-self-replicating mRNA formulation PDI, particle size

Non-replicating mRNA formulation encapsulation rate, mRNA purity
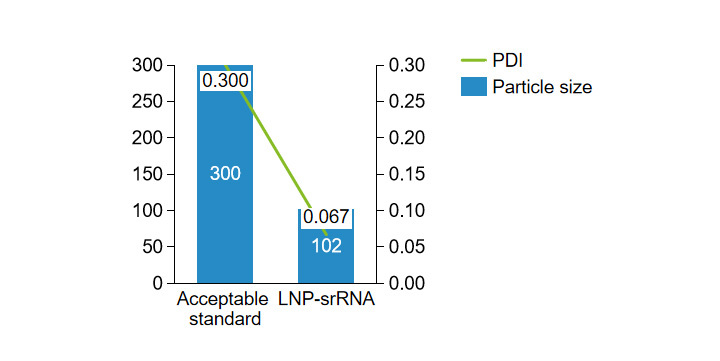
Self-replicating mRNA preparation PDI, particle size
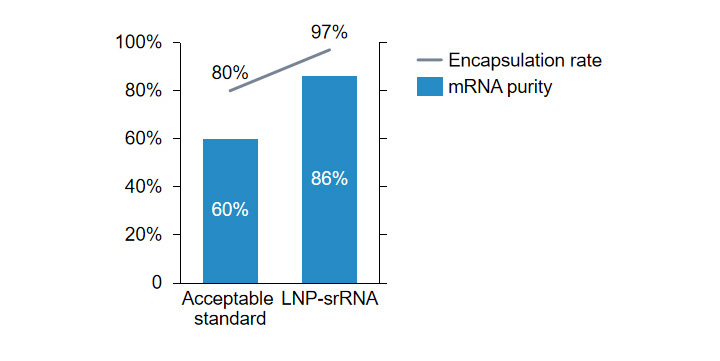
Self-replicating mRNA preparation wrapping rate, mRNA purity
|
Test item |
Test method |
|
Appearance |
Visual inspection |
|
pH |
Potentiometric method |
|
Load |
Weight |
|
Osmolality |
Freezing point method |
|
Particle Size |
Dynamic Light Scattering (DLS) |
|
PDI |
Dynamic Light Scattering (DLS) |
|
Zeta Potential Detection |
Phase Analytical Light Scattering (PALS) |
|
mRNA concentration |
HPLC/Ribogreen |
|
mRNA-LNP lipid concentration |
UPLC-UV/UPLC-ELSD |
|
Sequencing |
Sanger |
|
mRNA-LNP lipid identification |
UPLC-ELSD |
|
mRNA integrity |
CE/HPLC/Agarose gel |
|
Encapsulation rate |
Ribogreen |
|
Ethanol residue |
GC |
|
Sterility testing |
Pharmacopeial methods |
|
Endotoxin Detection |
Pharmacopeial Methods |
|
In vitro potency |
FACS/ELISA |
SAF Coolest v1.3.1.1 设置面板 AOOSX-AWNY-HASAE-ZAD
无数据提示
Sorry, the current column is being updated, please look forward to it!
You can view other columns or returnHome Page


