RNA technology
Own patented liquid dosage form heat stable delivery system, RTU LNP technology, has realized the stability verification of 2-8 ℃ storage for more than 18 months
Non-self-replicating mRNA stock solution
Own patented liquid dosage form heat stable delivery system, RTU LNP technology, has realized the stability verification of 2-8 ℃ storage for more than 18 months
The RNA Platform Case
Traditional non-self-made replication RNA (we ourselves are enzymatic, enzymatic to 100%)
> The capping rate is stable over 98%;
> mRNA purity over 90%;
> Maintaining good batch-to-batch consistency

Non-self-replicating mRNA stock mRNA purity, capping rate, Poly(A) tail length distribution
|
Test Items |
Test Methods |
|
Appearance |
Visual inspection |
|
pH |
Potentiometry |
|
mRNA concentration |
UV/Ribogreen |
|
5' capping efficiency |
LC-MS |
|
Poly A tail distribution |
LC-MS/CE |
|
mRNA integrity |
CE/HPLC/Agarose gel |
|
Sequencing |
Sanger |
|
dsRNA residues |
ELISA/Dot-blot |
|
IVTase residue (total protein) |
ELISA/NanoOrange |
|
DNA template residue detection |
ddPCR |
|
Sterility testing |
Pharmacopeial methods |
|
Endotoxin Testing |
Pharmacopeial methods |
Non-self-replicating mRNA preparations
Own patented liquid dosage form heat stable delivery system, RTU LNP technology, has realized the stability verification of 2-8 ℃ storage for more than 18 months
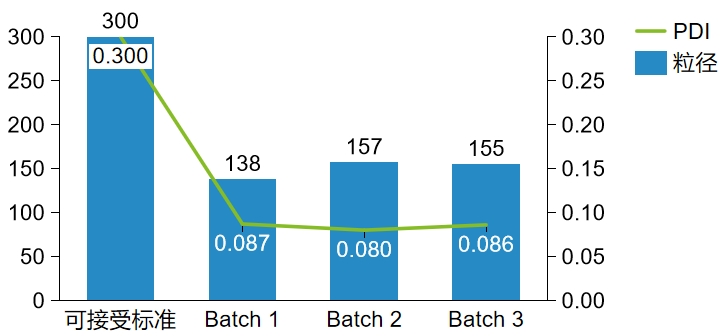
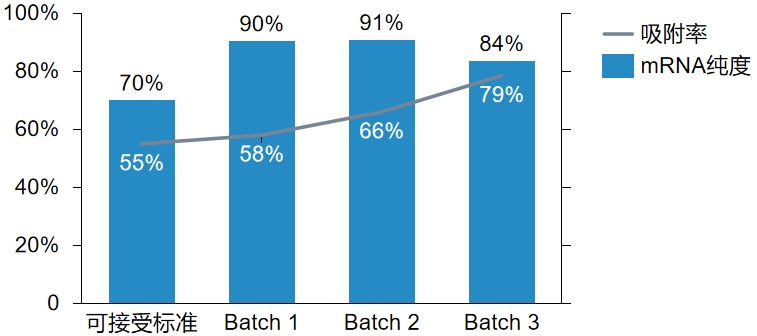
|
Test item |
Test method |
|
Appearance |
Visual inspection |
|
pH |
Potentiometric method |
|
Load |
Weight |
|
Osmolality |
Freezing point method |
|
Particle Size |
Dynamic Light Scattering (DLS) |
|
PDI |
Dynamic Light Scattering (DLS) |
|
Zeta Potential Detection |
Phase Analytical Light Scattering (PALS) |
|
mRNA concentration |
HPLC/Ribogreen |
|
mRNA-LNP lipid concentration |
UPLC-UV/UPLC-ELSD |
|
Sequencing |
Sanger |
|
mRNA-LNP lipid identification |
UPLC-ELSD |
|
mRNA integrity |
CE/HPLC/Agarose gel |
|
Encapsulation rate |
Ribogreen |
|
Ethanol residue |
GC |
|
Sterility testing |
Pharmacopeial methods |
|
Endotoxin Detection |
Pharmacopeial Methods |
|
In vitro potency |
FACS/ELISA |
Service guarantee

Project Experience
1. Complete 1 phase 1/2 clinical drug supply, and obtain 4 FDA IND approvals and 2 CDE IND approvals in total
2. Cumulative completion of 40 Tox & GMP batches
3. Service customers covering institutions, Biotech and well-known pharmaceutical companies

intellectual property protection
1. A clear confidentiality agreement to protect the customer's intellectual property rights;
2. Improve the project management mechanism to reduce project risk;
3. Strict account three-level authority management to effectively guarantee equipment operation authority;
4. Stable electronic data storage media, to achieve rights management, effectively guarantee the security and integrity of the data life cycle;
5. Standard IT technology methods realize physical isolation between office network and production network, control of computer USB rights, management of employees' online behavior, and restriction of users' query, copy and export of customer information.

Quality Management
1. According to ICH guidelines and FDA, EMA, NMPA and other requirements, a perfect quality system conforming to GMP requirements has been established, and more than 350 existing SOP and batch production record documents have been controlled.
2. Established a sound supplier management system
3. The standard EMS monitoring system can monitor the stability of key parameters such as temperature, humidity and pressure difference in the production workshop, clean room or storage facilities in real time to ensure the stability and safety of the production environment.
4. Standard computerized validation and verification system
SAF Coolest v1.3.1.1 设置面板 AOOSX-AWNY-HASAE-ZAD
无数据提示
Sorry, the current column is being updated, please look forward to it!
You can view other columns or returnHome Page


